Find the Concentration of a Solution Given Ksp
17.4: Solubility Equilibria
-
- Last updated
- Save as PDF
- Page ID
- 25186
Learning Objectives
- To calculate the solubility of an ionic compound from its K sp
We begin our discussion of solubility and complexation equilibria—those associated with the formation of complex ions—by developing quantitative methods for describing dissolution and precipitation reactions of ionic compounds in aqueous solution. Just as with acid–base equilibria, we can describe the concentrations of ions in equilibrium with an ionic solid using an equilibrium constant expression.
The Solubility Product
When a slightly soluble ionic compound is added to water, some of it dissolves to form a solution, establishing an equilibrium between the pure solid and a solution of its ions. For the dissolution of calcium phosphate, one of the two main components of kidney stones, the equilibrium can be written as follows, with the solid salt on the left:
\[Ca_3(PO_4)_{2(s)} \rightleftharpoons 3Ca^{2+}_{(aq)} + 2PO^{3−}_{4(aq)} \label{Eq1}\]
As you will discover in Section 17.4 and in more advanced chemistry courses, basic anions, such as S2−, PO4 3 −, and CO3 2 −, react with water to produce OH− and the corresponding protonated anion. Consequently, their calculated molarities, assuming no protonation in aqueous solution, are only approximate.
The equilibrium constant for the dissolution of a sparingly soluble salt is the solubility product (K sp) of the salt. Because the concentration of a pure solid such as Ca3(PO4)2 is a constant, it does not appear explicitly in the equilibrium constant expression. The equilibrium constant expression for the dissolution of calcium phosphate is therefore
\[K=\dfrac{[\mathrm{Ca^{2+}}]^3[\mathrm{PO_4^{3-}}]^2}{[\mathrm{Ca_3(PO_4)_2}]} \label{Eq2a}\]
\[[\mathrm{Ca_3(PO_4)_2}]K=K_{\textrm{sp}}=[\mathrm{Ca^{2+}}]^3[\mathrm{PO_4^{3-}}]^2 \label{Eq2b}\]
At 25°C and pH 7.00, Ksp for calcium phosphate is 2.07 × 10−33, indicating that the concentrations of Ca2 + and PO4 3 − ions in solution that are in equilibrium with solid calcium phosphate are very low. The values of K sp for some common salts are listed in Table \(\PageIndex{1}\), which shows that the magnitude of Ksp varies dramatically for different compounds. Although Ksp is not a function of pH in Equations \(\ref{Eq2a}\) and \(\ref{Eq2b}\), changes in pH can affect the solubility of a compound as discussed later.
As with any K, the concentration of a pure solid does not appear explicitly in K sp.
| Solid | Color | \(K_{sp}\) | Solid | Color | \(K_{sp}\) | |
|---|---|---|---|---|---|---|
| *These contain the Hg2 2 + ion. | ||||||
| Acetates | Iodides | |||||
| Ca(O2CCH3)2·3H2O | white | 4 × 10−3 | Hg2I2* | yellow | 5.2 × 10−29 | |
| Bromides | PbI2 | yellow | 9.8 × 10−9 | |||
| AgBr | off-white | 5.35 × 10−13 | Oxalates | |||
| Hg2Br2* | yellow | 6.40 × 10−23 | Ag2C2O4 | white | 5.40 × 10−12 | |
| Carbonates | MgC2O4·2H2O | white | 4.83 × 10−6 | |||
| CaCO3 | white | 3.36 × 10−9 | PbC2O4 | white | 4.8 × 10−10 | |
| PbCO3 | white | 7.40 × 10−14 | Phosphates | |||
| Chlorides | Ag3PO4 | white | 8.89 × 10−17 | |||
| AgCl | white | 1.77 × 10−10 | Sr3(PO4)2 | white | 4.0 × 10−28 | |
| Hg2Cl2* | white | 1.43 × 10−18 | FePO4·2H2O | pink | 9.91 × 10−16 | |
| PbCl2 | white | 1.70 × 10−5 | Sulfates | |||
| Chromates | Ag2SO4 | white | 1.20 × 10−5 | |||
| CaCrO4 | yellow | 7.1 × 10−4 | BaSO4 | white | 1.08 × 10−10 | |
| PbCrO4 | yellow | 2.8 × 10−13 | PbSO4 | white | 2.53 × 10−8 | |
| Fluorides | Sulfides | |||||
| BaF2 | white | 1.84 × 10−7 | Ag2S | black | 6.3 × 10−50 | |
| PbF2 | white | 3.3 × 10−8 | CdS | yellow | 8.0 × 10−27 | |
| Hydroxides | PbS | black | 8.0 × 10−28 | |||
| Ca(OH)2 | white | 5.02 × 10−6 | ZnS | white | 1.6 × 10−24 | |
| Cu(OH)2 | pale blue | 1 × 10−14 | ||||
| Mn(OH)2 | light pink | 1.9 × 10−13 | ||||
| Cr(OH)3 | gray-green | 6.3 × 10−31 | ||||
| Fe(OH)3 | rust red | 2.79 × 10−39 | ||||
Definition of a Solubility Product: https://youtu.be/VzxSmH_iwHE
Solubility products are determined experimentally by directly measuring either the concentration of one of the component ions or the solubility of the compound in a given amount of water. However, whereas solubility is usually expressed in terms of mass of solute per 100 mL of solvent, \(K_{sp}\), like \(K\), is defined in terms of the molar concentrations of the component ions.

Example \(\PageIndex{1}\)
Calcium oxalate monohydrate [Ca(O2CCO2)·H2O, also written as CaC2O4·H2O] is a sparingly soluble salt that is the other major component of kidney stones [along with Ca3(PO4)2]. Its solubility in water at 25°C is 7.36 × 10−4 g/100 mL. Calculate its K sp.
Given: solubility in g/100 mL
Asked for: K sp
Strategy:
- Write the balanced dissolution equilibrium and the corresponding solubility product expression.
- Convert the solubility of the salt to moles per liter. From the balanced dissolution equilibrium, determine the equilibrium concentrations of the dissolved solute ions. Substitute these values into the solubility product expression to calculate K sp.
Solution
A We need to write the solubility product expression in terms of the concentrations of the component ions. For calcium oxalate monohydrate, the balanced dissolution equilibrium and the solubility product expression (abbreviating oxalate as ox2 −) are as follows:
\(\mathrm{Ca(O_2CCO_2)}\cdot\mathrm{H_2O(s)}\rightleftharpoons \mathrm{Ca^{2+}(aq)}+\mathrm{^-O_2CCO_2^-(aq)}+\mathrm{H_2O(l)}\hspace{5mm}K_{\textrm{sp}}=[\mathrm{Ca^{2+}}][\mathrm{ox^{2-}}]\)
Neither solid calcium oxalate monohydrate nor water appears in the solubility product expression because their concentrations are essentially constant.
B Next we need to determine [Ca2 +] and [ox2 −] at equilibrium. We can use the mass of calcium oxalate monohydrate that dissolves in 100 mL of water to calculate the number of moles that dissolve in 100 mL of water. From this we can determine the number of moles that dissolve in 1.00 L of water. For dilute solutions, the density of the solution is nearly the same as that of water, so dissolving the salt in 1.00 L of water gives essentially 1.00 L of solution. Because each 1 mol of dissolved calcium oxalate monohydrate dissociates to produce 1 mol of calcium ions and 1 mol of oxalate ions, we can obtain the equilibrium concentrations that must be inserted into the solubility product expression. The number of moles of calcium oxalate monohydrate that dissolve in 100 mL of water is as follows:
\(\dfrac{7.36\times10^{-4}\textrm{ g}}{146.1\textrm{ g/mol}}=5.04\times10^{-6}\textrm{ mol }\mathrm{Ca(O_2CCO_2)\cdot H_2O}\)
The number of moles of calcium oxalate monohydrate that dissolve in 1.00 L of the saturated solution is as follows:
\(\left(\dfrac{5.04\times10^{-6}\textrm{ mol }\mathrm{Ca(O_2CCO_2\cdot)H_2O}}{\textrm{100 mL}}\right)\left(\dfrac{\textrm{1000 mL}}{\textrm{1.00 L}}\right)=5.04\times10^{-5}\textrm{ mol/L}=5.04\times10^{-5}\textrm{ M}\)
Because of the stoichiometry of the reaction, the concentration of Ca2 + and ox2 − ions are both 5.04 × 10−5 M. Inserting these values into the solubility product expression,
\[K_{sp} = [Ca^{2+}][ox^{2−}] = (5.04 \times 10^{−5})(5.04 \times10^{−5}) = 2.54 \times 10^{−9}\]
In our calculation, we have ignored the reaction of the weakly basic anion with water, which tends to make the actual solubility of many salts greater than the calculated value.
Exercise \(\PageIndex{1}\): Calcite
One crystalline form of calcium carbonate (CaCO3) is "calcite", found as both a mineral and a structural material in many organisms. Calcite is found in the teeth of sea urchins. The urchins create depressions in limestone that they can settle in by grinding the rock with their teeth. Limestone, however, also consists of calcite, so how can the urchins grind the rock without also grinding their teeth? Researchers have discovered that the teeth are shaped like needles and plates and contain magnesium. The concentration of magnesium increases toward the tip, which contributes to the hardness. Moreover, each tooth is composed of two blocks of the polycrystalline calcite matrix that are interleaved near the tip. This creates a corrugated surface that presumably increases grinding efficiency. Toolmakers are particularly interested in this approach to grinding.

The solubility of calcite in water is 0.67 mg/100 mL. Calculate its K sp.
Answer
4.5 × 10−9
The reaction of weakly basic anions with H2O tends to make the actual solubility of many salts higher than predicted.
Finding Ksp from Ion Concentrations: https://youtu.be/a8nhlJk8UX0
Tabulated values of K sp can also be used to estimate the solubility of a salt with a procedure that is essentially the reverse of the one used in Example \(\PageIndex{1}\). In this case, we treat the problem as a typical equilibrium problem and set up a table of initial concentrations, changes in concentration, and final concentrations (ICE Tables), remembering that the concentration of the pure solid is essentially constant.
Example \(\PageIndex{2}\)
We saw that the K sp for Ca3(PO4)2 is 2.07 × 10−33 at 25°C. Calculate the aqueous solubility of Ca3(PO4)2 in terms of the following:
- the molarity of ions produced in solution
- the mass of salt that dissolves in 100 mL of water at 25°C
Given: K sp
Asked for: molar concentration and mass of salt that dissolves in 100 mL of water
Strategy:
- Write the balanced equilibrium equation for the dissolution reaction and construct a table showing the concentrations of the species produced in solution. Insert the appropriate values into the solubility product expression and calculate the molar solubility at 25°C.
- Calculate the mass of solute in 100 mL of solution from the molar solubility of the salt. Assume that the volume of the solution is the same as the volume of the solvent.
Solution:
- A The dissolution equilibrium for Ca3(PO4)2 (Equation \(\ref{Eq2a}\)) is shown in the following ICE table. Because we are starting with distilled water, the initial concentration of both calcium and phosphate ions is zero. For every 1 mol of Ca3(PO4)2 that dissolves, 3 mol of Ca2 + and 2 mol of PO4 3 − ions are produced in solution. If we let x equal the solubility of Ca3(PO4)2 in moles per liter, then the change in [Ca2 +] will be +3x, and the change in [PO4 3−] will be +2x. We can insert these values into the table.
Ca3(PO4)2(s) ⇌ 3Ca2 +(aq) + 2PO4 3−(aq)
| Ca3(PO4)2 | [Ca2+] | [PO4 3−] | |
|---|---|---|---|
| initial | pure solid | 0 | 0 |
| change | — | +3x | +2x |
| final | pure solid | 3x | 2x |
Although the amount of solid Ca3(PO4)2 changes as some of it dissolves, its molar concentration does not change. We now insert the expressions for the equilibrium concentrations of the ions into the solubility product expression (Equation 17.2):
\(\begin{align}K_{\textrm{sp}}=[\mathrm{Ca^{2+}}]^3[\mathrm{PO_4^{3-}}]^2&=(3x)^3(2x)^2
\\2.07\times10^{-33}&=108x^5
\\1.92\times10^{-35}&=x^5
\\1.14\times10^{-7}\textrm{ M}&=x\end{align}\)
This is the molar solubility of calcium phosphate at 25°C. However, the molarity of the ions is 2x and 3x, which means that [PO4 3−] = 2.28 × 10−7 and [Ca2 +] = 3.42 × 10−7.
- B To find the mass of solute in 100 mL of solution, we assume that the density of this dilute solution is the same as the density of water because of the low solubility of the salt, so that 100 mL of water gives 100 mL of solution. We can then determine the amount of salt that dissolves in 100 mL of water:
\(\left(\dfrac{1.14\times10^{-7}\textrm{ mol}}{\textrm{1 L}}\right)\textrm{100 mL}\left(\dfrac{\textrm{1 L}}{\textrm{1000 mL}} \right )\left(\dfrac{310.18 \textrm{ g }\mathrm{Ca_3(PO_4)_2}}{\textrm{1 mol}}\right)=3.54\times10^{-6}\textrm{ g }\mathrm{Ca_3(PO_4)_2}\)
Exercise \(\PageIndex{2}\)
The solubility product of silver carbonate (Ag2CO3) is 8.46 × 10−12 at 25°C. Calculate the following:
- the molarity of a saturated solution
- the mass of silver carbonate that will dissolve in 100 mL of water at this temperature
Answer
- 1.28 × 10−4 M
- 3.54 mg
The Ion Product
The ion product (Q) of a salt is the product of the concentrations of the ions in solution raised to the same powers as in the solubility product expression. It is analogous to the reaction quotient (Q) discussed for gaseous equilibria. Whereas K sp describes equilibrium concentrations, the ion product describes concentrations that are not necessarily equilibrium concentrations.
The ion product Q is analogous to the reaction quotient Q for gaseous equilibria.
As summarized in Figure \(\PageIndex{1}\), there are three possible conditions for an aqueous solution of an ionic solid:
- Q < K sp. The solution is unsaturated, and more of the ionic solid, if available, will dissolve.
- Q = K sp. The solution is saturated and at equilibrium.
- Q > K sp. The solution is supersaturated, and ionic solid will precipitate.
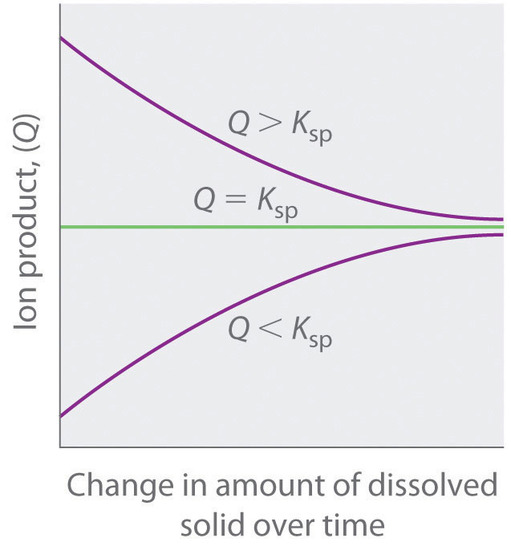
The process of calculating the value of the ion product and comparing it with the magnitude of the solubility product is a straightforward way to determine whether a solution is unsaturated, saturated, or supersaturated. More important, the ion product tells chemists whether a precipitate will form when solutions of two soluble salts are mixed.
Example \(\PageIndex{3}\)
We mentioned that barium sulfate is used in medical imaging of the gastrointestinal tract. Its solubility product is 1.08 × 10−10 at 25°C, so it is ideally suited for this purpose because of its low solubility when a "barium milkshake" is consumed by a patient. The pathway of the sparingly soluble salt can be easily monitored by x-rays. Will barium sulfate precipitate if 10.0 mL of 0.0020 M Na2SO4 is added to 100 mL of 3.2 × 10−4 M BaCl2? Recall that NaCl is highly soluble in water.
Given: K sp and volumes and concentrations of reactants
Asked for: whether precipitate will form
Strategy:
- Write the balanced equilibrium equation for the precipitation reaction and the expression for K sp.
- Determine the concentrations of all ions in solution when the solutions are mixed and use them to calculate the ion product (Q).
- Compare the values of Q and K sp to decide whether a precipitate will form.
Solution
A The only slightly soluble salt that can be formed when these two solutions are mixed is BaSO4 because NaCl is highly soluble. The equation for the precipitation of BaSO4 is as follows:
\[BaSO_{4(s)} \rightleftharpoons Ba^{2+}_{(aq)} + SO^{2−}_{4(aq)}\]
The solubility product expression is as follows:
K sp = [Ba2 +][SO4 2 −] = 1.08×10−10
B To solve this problem, we must first calculate the ion product—Q = [Ba2 +][SO4 2 −]—using the concentrations of the ions that are present after the solutions are mixed and before any reaction occurs. The concentration of Ba2 + when the solutions are mixed is the total number of moles of Ba2 + in the original 100 mL of BaCl2 solution divided by the final volume (100 mL + 10.0 mL = 110 mL):
\(\textrm{moles Ba}^{2+}=\textrm{100 mL}\left(\dfrac{\textrm{1 L}}{\textrm{1000 mL}}\right)\left(\dfrac{3.2\times10^{-4}\textrm{ mol}}{\textrm{1 L}} \right )=3.2\times10^{-5}\textrm{ mol Ba}^{2+}\)
\([\mathrm{Ba^{2+}}]=\left(\dfrac{3.2\times10^{-5}\textrm{ mol Ba}^{2+}}{\textrm{110 mL}}\right)\left(\dfrac{\textrm{1000 mL}}{\textrm{1 L}}\right)=2.9\times10^{-4}\textrm{ M Ba}^{2+}\)
Similarly, the concentration of SO4 2 − after mixing is the total number of moles of SO4 2 − in the original 10.0 mL of Na2SO4 solution divided by the final volume (110 mL):
\(\textrm{moles SO}_4^{2-}=\textrm{10.0 mL}\left(\dfrac{\textrm{1 L}}{\textrm{1000 mL}}\right)\left(\dfrac{\textrm{0.0020 mol}}{\textrm{1 L}}\right)=2.0\times10^{-5}\textrm{ mol SO}_4^{2-}\)
\([\mathrm{SO_4^{2-}}]=\left(\dfrac{2.0\times10^{-5}\textrm{ mol SO}_4^{2-}}{\textrm{110 mL}} \right )\left(\dfrac{\textrm{1000 mL}}{\textrm{1 L}}\right)=1.8\times10^{-4}\textrm{ M SO}_4^{2-}\)
We can now calculate Q:
Q = [Ba2 +][SO4 2 −] = (2.9×10−4)(1.8×10−4) = 5.2×10−8
C We now compare Q with the K sp. If Q > K sp, then BaSO4 will precipitate, but if Q < K sp, it will not. Because Q > K sp, we predict that BaSO4 will precipitate when the two solutions are mixed. In fact, BaSO4 will continue to precipitate until the system reaches equilibrium, which occurs when [Ba2 +][SO4 2 −] = K sp = 1.08 × 10−10.
Exercise \(\PageIndex{3}\)
The solubility product of calcium fluoride (CaF2) is 3.45 × 10−11. If 2.0 mL of a 0.10 M solution of NaF is added to 128 mL of a 2.0 × 10−5M solution of Ca(NO3)2, will CaF2 precipitate?
- Answer
-
yes (Q = 4.7 × 10−11 > K sp)
Determining if a Precipitate forms (The Ion Product): https://youtu.be/Naf7PoHPz8Y
The Common Ion Effect and Solubility
The solubility product expression tells us that the equilibrium concentrations of the cation and the anion are inversely related. That is, as the concentration of the anion increases, the maximum concentration of the cation needed for precipitation to occur decreases—and vice versa—so that K sp is constant. Consequently, the solubility of an ionic compound depends on the concentrations of other salts that contain the same ions. Adding a common cation or anion shifts a solubility equilibrium in the direction predicted by Le Chatelier's principle. As a result, the solubility of any sparingly soluble salt is almost always decreased by the presence of a soluble salt that contains a common ion. The exceptions generally involve the formation of complex ions, which is discussed later.
Consider, for example, the effect of adding a soluble salt, such as CaCl2, to a saturated solution of calcium phosphate [Ca3(PO4)2]. We have seen that the solubility of Ca3(PO4)2 in water at 25°C is 1.14 × 10−7 M (K sp = 2.07 × 10−33). Thus a saturated solution of Ca3(PO4)2 in water contains 3 × (1.14 × 10−7 M) = 3.42 × 10−7 M Ca2 + and 2 × (1.14 × 10−7 M) = 2.28 × 10−7 M PO4 3 −, according to the stoichiometry shown in Equation \(\ref{Eq1}\) (neglecting hydrolysis to form HPO4 2 − as described in Chapter 16). If CaCl2 is added to a saturated solution of Ca3(PO4)2, the Ca2 + ion concentration will increase such that [Ca2 +] > 3.42 × 10−7 M, making Q > K sp. The only way the system can return to equilibrium is for the reaction in Equation \(\ref{Eq1}\) to proceed to the left, resulting in precipitation of Ca3(PO4)2. This will decrease the concentration of both Ca2 + and PO4 3 − until Q = K sp.
The common ion effect usually decreases the solubility of a sparingly soluble salt.
Example \(\PageIndex{4}\)
Calculate the solubility of calcium phosphate [Ca3(PO4)2] in 0.20 M CaCl2.
Given: concentration of CaCl2 solution
Asked for: solubility of Ca3(PO4)2 in CaCl2 solution
Strategy:
- Write the balanced equilibrium equation for the dissolution of Ca3(PO4)2. Tabulate the concentrations of all species produced in solution.
- Substitute the appropriate values into the expression for the solubility product and calculate the solubility of Ca3(PO4)2.
Solution
A The balanced equilibrium equation is given in the following table. If we let x equal the solubility of Ca3(PO4)2 in moles per liter, then the change in [Ca2 +] is once again +3x, and the change in [PO4 3 −] is +2x. We can insert these values into the ICE table.
\[Ca_3(PO_4)_{2(s)} \rightleftharpoons 3Ca^{2+}_{(aq)} + 2PO^{3−}_{4(aq)} \nonumber\]
| Ca3(PO4)2 | [Ca2+] | [PO4 3−] | |
|---|---|---|---|
| initial | pure solid | 0.20 | 0 |
| change | — | +3x | +2x |
| final | pure solid | 0.20 + 3x | 2x |
B The K sp expression is as follows:
K sp = [Ca2 +]3[PO4 3 −]2 = (0.20 + 3x)3(2x)2 = 2.07×10−33
Because Ca3(PO4)2 is a sparingly soluble salt, we can reasonably expect that x << 0.20. Thus (0.20 + 3x) M is approximately 0.20 M, which simplifies the K sp expression as follows:
\[\begin{align*}K_{\textrm{sp}}=(0.20)^3(2x)^2&=2.07\times10^{-33}
\\x^2&=6.5\times10^{-32}
\\x&=2.5\times10^{-16}\textrm{ M}\end{align*}\]
This value is the solubility of Ca3(PO4)2 in 0.20 M CaCl2 at 25°C. It is approximately nine orders of magnitude less than its solubility in pure water, as we would expect based on Le Chatelier's principle. With one exception, this example is identical to Example \(\PageIndex{2}\)—here the initial [Ca2 +] was 0.20 M rather than 0.
Exercise \(\PageIndex{4}\)
Calculate the solubility of silver carbonate in a 0.25 M solution of sodium carbonate. The solubility of silver carbonate in pure water is 8.45 × 10−12 at 25°C.
- Answer
-
2.9 × 10−6 M (versus 1.3 × 10−4 M in pure water)
Summary
The solubility product (K sp) is used to calculate equilibrium concentrations of the ions in solution, whereas the ion product (Q) describes concentrations that are not necessarily at equilibrium. The equilibrium constant for a dissolution reaction, called the solubility product (K sp), is a measure of the solubility of a compound. Whereas solubility is usually expressed in terms of mass of solute per 100 mL of solvent, K sp is defined in terms of the molar concentrations of the component ions. In contrast, the ion product (Q) describes concentrations that are not necessarily equilibrium concentrations. Comparing Q and K sp enables us to determine whether a precipitate will form when solutions of two soluble salts are mixed. Adding a common cation or common anion to a solution of a sparingly soluble salt shifts the solubility equilibrium in the direction predicted by Le Chatelier's principle. The solubility of the salt is almost always decreased by the presence of a common ion.
Find the Concentration of a Solution Given Ksp
Source: https://chem.libretexts.org/Bookshelves/General_Chemistry/Map%3A_Chemistry_-_The_Central_Science_(Brown_et_al.)/17%3A_Additional_Aspects_of_Aqueous_Equilibria/17.4%3A_Solubility_Equilibria